July 24, 2018 – Status on Over the Counter (OTC) Hearing Aids
In the FDA Reauthorization Act of 2017 (FDARA), Congress outlined certain requirements to establish a category of over-the-counter (OTC) hearing aids and the requirements that apply to them. This statutorily mandated process requires FDA to publish proposed regulations for public comment, and then to publish final regulations.
At this time, there are no products that can claim to address hearing loss that are, or can claim to be OTC hearing aids within the meaning of section 520(q) of the FD&C Act as amended by FDARA. Currently, hearing aids continue to be restricted devices, for which sales must follow applicable federal and state requirements. FDA has published a letter to clarify the status of these products.
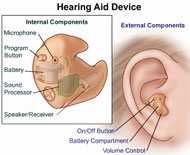
Having trouble hearing? Over 35 million children and adults in the United States have some degree of hearing loss. Hearing loss can have a negative effect on communication, relationships, school/work performance, and emotional well-being. However, hearing loss doesn’t have to restrict your daily activities. Properly fitted hearing aids and aural rehabilitation (techniques used to identify and diagnose hearing loss, and implement therapies for patients who are hard of hearing, including using amplification devices to aid the patient’s hearing abilities), can help in many listening situations. Aural rehabilitation helps a person focus on adjusting to their hearing loss and the use of their hearing aids. It also explores assistive devices to help improve communication. Most people who are hearing-impaired will need two hearing aids as both ears may be affected by hearing loss, though some people may only need one hearing aid.
This site includes information on the difference between hearing aids, intended for use by people with hearing loss, and sound amplifiers for consumers with no hearing loss who want to make environmental sounds louder for recreational use. While the FDA regulates hearing aids, which are medical devices, it does not consider sound amplifiers to be medical devices when labeled for recreational or other use by individuals with normal hearing. However, certain safety regulations related to sound output levels still apply to these products.
Recently, the President’s Council of Advisors on Science and Technology (PCAST), and the National Academies of Sciences, Engineering and Medicine (NAS) issued reports recommending ways to improve the access and affordability of hearing aids. The FDA considered these recommendations along with input from the public and has issued the “Immediately in Effect Guidance Document: Conditions for Sale for Air-Conduction Hearing Aids” guidance document. The FDA issued this guidance to communicate that it does not intend to enforce certain conditions for sale applicable to hearing aids for users 18 years of age or older.
This site provides general information on hearing aids and their benefits, types of hearing loss, other products and procedures to improve hearing, and a checklist of steps to remember and consider before purchasing hearing aids. This site is not intended to provide medical advice. If you have questions about your health, the best source of information is your hearing health care professional.
